
研究概要
Society5.0が目指す近未来の対がん医療体制では、QOL(Quality of Life)を重視した非侵襲的な診断・治療技術が求められています。その実現には、がん細胞内生化学反応と臨床症例との関連解析のためのビッグデータを得る必要があります。従って、そのための革新的な材料開発が強く望まれています。今日では、力学作用の一つである衝撃波や超音波などの音響波を用いたがん細胞の生物学的応答の可視化、およびがん細胞を攻撃(治療)するための新材料の開発が注目されています。
我々の研究室では、細胞に実際にかかる圧力(音響波)を計測可能な革新的な光機能ソフトマテリアル(有機~超分子~高分子)の探索・開発を行っています。
The near-future cancer medical system envisioned by Society 5.0 requires non-invasive diagnostic and treatment technologies that emphasize quality of life (QOL). To achieve this, it is necessary to obtain big data for analyzing the correlation between biochemical reactions within cancer cells and clinical cases. Therefore, the development of innovative materials for this purpose is highly desirable. Currently, attention is being paid to the visualization of the biological responses of cancer cells using mechanical effects such as shock waves and acoustic waves, as well as the development of new materials for attacking (treating) cancer cells. Our laboratory is exploring and developing innovative photofunctional soft materials (organic, supramolecular, and polymeric) that can measure the actual pressure (acoustic waves) exerted on cells.

テーマ
<増幅計測グループ(高感度腫瘍マーカー)>
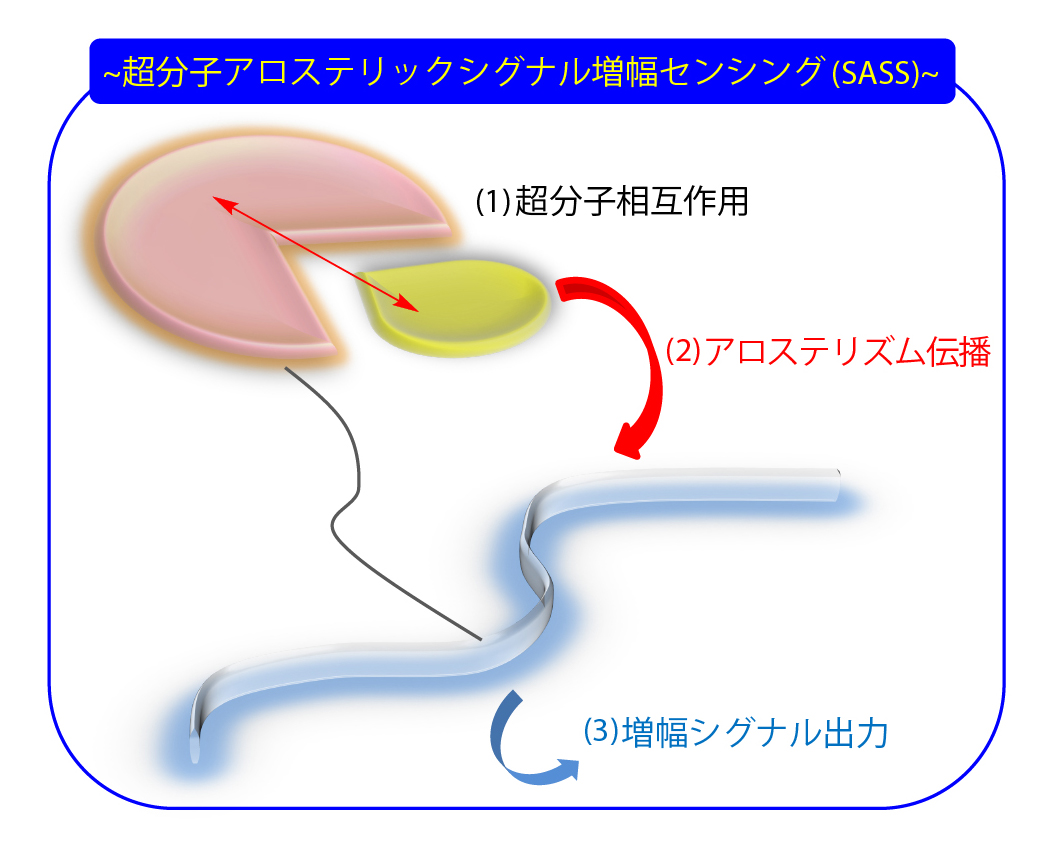
我々は2015年に、新規な増幅計測手法を提唱しました。生体系におけるアロステリック効果を利用することで、(1)精密に設計された受容体での検体の吸着・包接に伴うセンシング部位自身の動的構造変化により、(2)アロステリック効果によってシグナル増幅部位への伝播を引き起こさせ、(3)ここから増幅したシグナルを得るという手法を提唱しています。そして、この一連のプロセスを利用するセンシング方法論を“超分子アロステリックシグナル増幅センシング(Supramolecular Allosteric Signal-amplification Sensing)” (SASS)と定義して、幅広い生体関連物質に展開しています1-4。例えば、膵臓がんが分泌するペプチドに対しても高感度センシングが可能であることから、医療診断材料として期待されています5,6。
<Amplification Measurement Group (Highly Sensitive Tumor Markers)>
In 2015, we proposed a novel amplification measurement method. By utilizing allosteric effects in biological systems, (1) the adsorption and encapsulation of analytes by precisely designed receptors induces dynamic structural changes at the sensing site itself, (2) triggering allosteric propagation to the signal amplification site, and (3) obtaining an amplified signal. We have defined this sensing methodology, called “Supramolecular Allosteric Signal-amplification Sensing” (SASS), and are applying it to a wide range of biologically relevant substances. 1-4. For example, our method is capable of highly sensitively sensing peptides secreted by pancreatic cancer, making it promising for use in medical diagnostics. 5,6
参考文献
- Fukuhara, G.*: Polymer-Based Supramolecular Sensing and Application to Chiral Photochemistry. Polym. J. 2015, 47, 649-655. [Focus Review]
- Fukuhara, G.*: Allosteric signal-amplification sensing with polymer-based supramolecular hosts. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 127-143. [Review]
- Fukuhara, G.*: Analytical supramolecular chemistry: colorimetric and fluorimetric chemosensors. J. Photochem. Photobiol. C: Photochem. Rev. 2020, 42, 100340. [Review]
- Fukuhara, G.*: Smart polymer chemosensors: Signal-amplification systems with allosterism. Polym. J. 2021, 53, 1325-1334. [Review]
- Nishi, R.; Ishida, Y.; Mizuno, H.; Kawauchi, S.; Fukuhara, G.*: Allosteric Signal-Amplification Sensing of Peptides with Cyclodextrin-Polymer Conjugates in Aqueous Media, ACS Appl. Polym. Mater. 2023, 5, 3653-3660. [Supplementary Cover]
- Mizuno, H.; Nakazawa, H.; Miyagawa, A.; Yakiyama, Y.; Sakurai, H.*; Fukuhara, G.*: Amplification sensing manipulated by a sumanene-based supramolecular polymer as a dynamic allosteric effector, Sci. Rep. 2024, 14, 12534. [Press Release] [Eurekalert!]
<併用療法グループ>
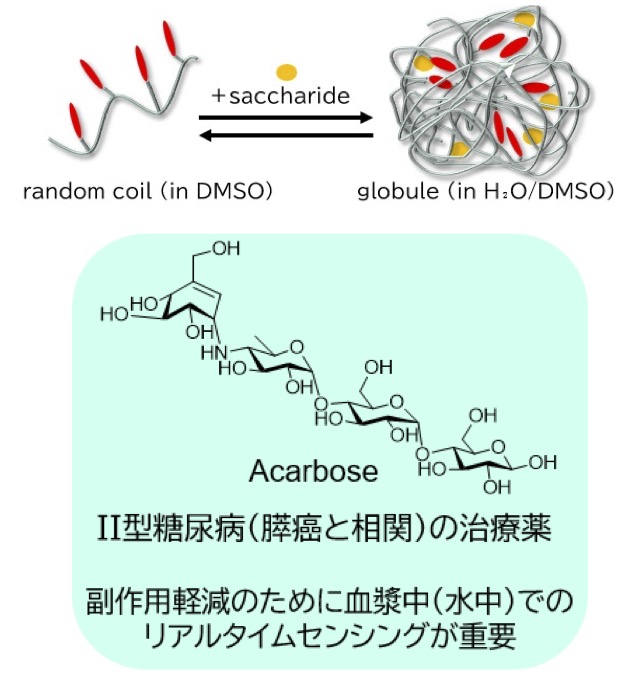
併用療法とは、有効性や抗腫瘍メカニズムが異なる化学療法剤を組合わせて用いる薬物療法です。本プロジェクトでは、がんと糖をターゲットとした併用療法です。近年、Ⅱ型糖尿病と種々のがんの発症に相関があることが示唆されています。Ⅱ型糖尿病の治療薬の一つとして、アカルボースが挙げられます。アカルボースは血糖値の上昇を抑える効果があり、Ⅱ型糖尿病や肥満の治療薬として世界各国で広く使用されています。しかし、過剰に摂取すると低血糖を引き起こす可能性があり、不足すると高血糖を招くことがあります。がん患者においては、高血糖状態が脱水や感染リスクを増大させる一方、低血糖状態では合併症のリスクが高まるため、がん治療と並行して血糖値を適切に管理することが非常に重要です。そのため、血糖値のリアルタイムモニタリングが不可欠とされています。
我々の研究室では、多糖のカードラン(Cur)にシグナル出力リポーターを導入した修飾Curセンサー(random coil)がアカルボースを空孔内に取り込むことで(globule)、定量的かつ高感度(μMオーダー)にセンシングできることを見出しています1-6。
<Combination Therapy Group>
Combination therapy is a drug therapy that combines chemotherapy agents with different efficacies and antitumor mechanisms. This project focuses on combination therapy targeting cancer and glucose. In recent years, a correlation between type 2 diabetes and various cancers has been suggested. Acarbose is one of the drugs used to treat type 2 diabetes. Acarbose suppresses blood glucose levels and is widely used worldwide as a treatment for type 2 diabetes and obesity. However, excessive intake can cause hypoglycemia, while insufficient intake can lead to hyperglycemia. In cancer patients, hyperglycemia increases the risk of dehydration and infection, while hypoglycemia increases the risk of complications. Therefore, appropriate blood glucose management is crucial in parallel with cancer treatment. Therefore, real-time monitoring of blood glucose levels is essential. Our laboratory has discovered that a modified curdlan (Cur) sensor (random coil), in which a signal output reporter is introduced into the polysaccharide Cur, can quantitatively and sensitively sense acarbose (on the order of μM) by incorporating it into its globules. 1-6
参考文献
- Fukuhara, G.*; Inoue, Y.*: Oligosaccharide sensing with chromophore-modified curdlan in aqueous media. Chem. Commun. 2010, 46, 9128-9130. [Hot Article]
- Fukuhara, G.*; Inoue, Y.*: Highly Selective Oligosaccharide Sensing by a Curdlan-Polythiophene Hybrid. J. Am. Chem. Soc. 2011, 133, 768-770.
- Fukuhara, G.*; Sasaki, M.; Numata, M.; Mori, T.; Inoue, Y.*: Oligosaccharide Sensing in Aqueous Media by Porphyrin-Curdlan Conjugates: A Prêt-á-Porter Rather Than Haute-Couture Approach. Chem. Eur. J. 2017, 23, 11272-11278. [Inside Cover]
- Sasaki, M.; Ryoson, Y.; Numata, M.; Fukuhara, G.*: Oligosaccharide Sensing in Aqueous Media Using Porphyrin-Curdlan Conjugates: An Allosteric Signal-Amplification System. J. Org. Chem. 2019, 84, 6017-6027. [ACS Editors’ Choice] [Supplementary Cover]
- Kurohara, H.; Hori, Y.; Numata, M.; Fukuhara, G.*: Oligosaccharide Sensing Using Fluorophore-Probed Curdlans in Aqueous Media, ACS Appl. Polym. Mater. 2023, 5, 2254-2263.
- Norikuni, M.; Hori, Y.; Numata, M.; Matsusaki, M.; Kida, T.; Fukuhara, G.*: Fluorophore-Probed Curdlan Polysaccharide Chemosensor: “Turn-On” Oligosaccharide Sensing in Aqueous Media, ACS Omega 2024, 9, 22345-22351.
<音響波イメージンググループ>
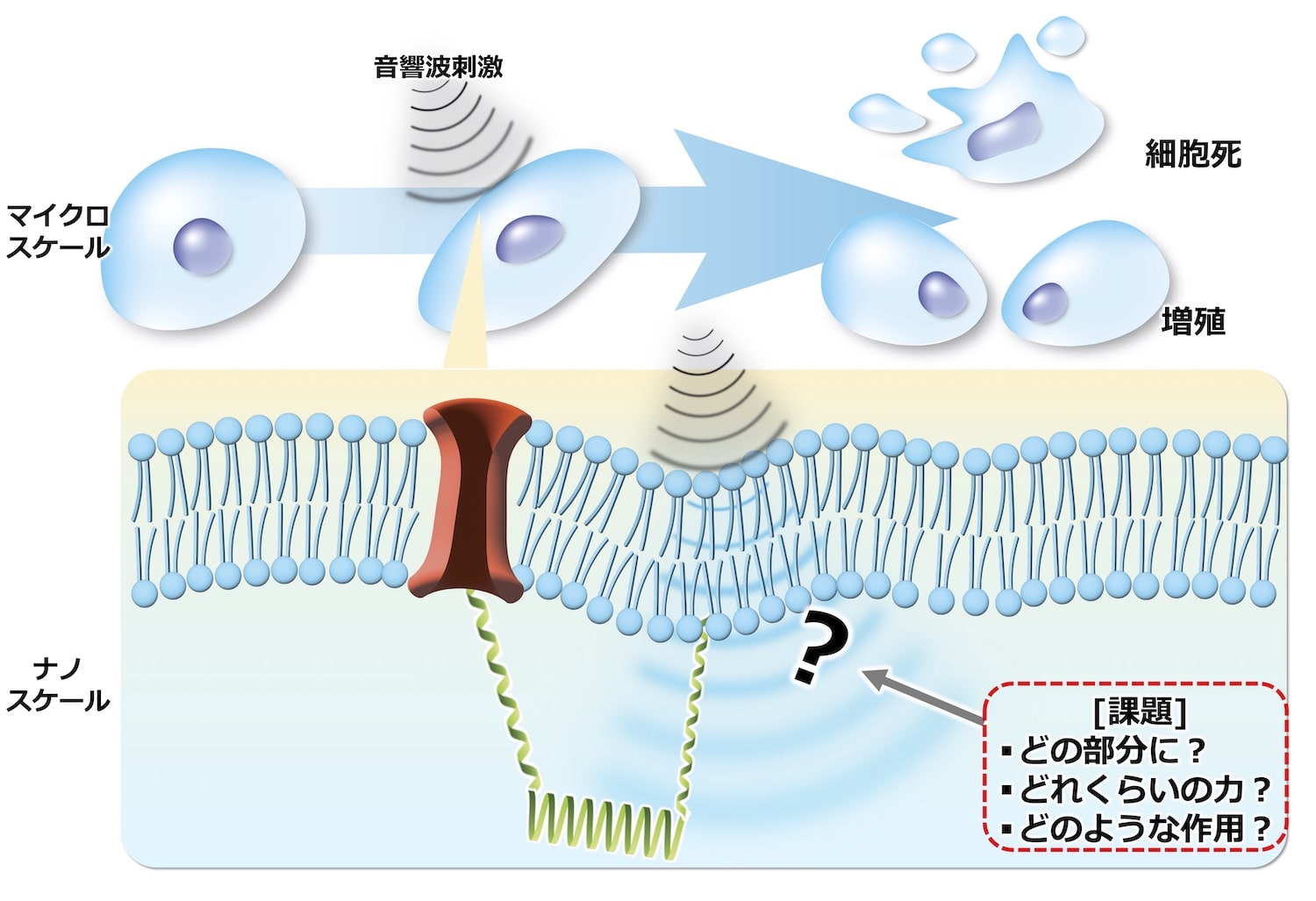
近年注目されているのは、力学作用の一つである衝撃波などの音響波(圧力波の伝播)を用いたがん細胞の生物学的応答、さらには細胞内での生化学反応を可視化するための新診断材料の開発です。しかし、現在の技術ではマイクロスケールでの細胞応答と、ナノ分子レベルの生化学反応の相関を解析する手法が未確立であり、材料開発の大きな障壁となっています。実際、現在のメカノバイオロジーにおいて「細胞のどの部分に?」「どれ位の力が働き?」「どのような生化学反応を誘起しているのか?」については未だに「観ること」ができていません。これは、細胞に実際にかかる圧力(音響波)を計測できるプローブが30年以上実現できなかったためです。この音響波観測手法の致命的問題点を解決しうる材料として、我々の研究室では「感圧ソフトマテリアル」(圧力を分子の発光として読み出す分子材料)という医療診断に資する物質を発見しました1。現在、実際に細胞にかかる圧力波をイメージングできる感圧ソフトマテリアルの大規模探索・開発を行っています2-6。
<Acoustic Wave Imaging Group>
In recent years, attention has focused on the development of new diagnostic materials for visualizing the biological responses of cancer cells and biochemical reactions within cells using acoustic waves (pressure wave propagation), such as shock waves, a type of mechanical action. However, current technology lacks a method for analyzing the correlation between microscale cellular responses and nanomolecular-level biochemical reactions, posing a major obstacle to material development. In fact, current mechanobiology has yet to “observe” the following questions: “Which part of the cell?”, “How much force is acting?”, and “What kind of biochemical reactions are being induced?” This is because, for over 30 years, a probe capable of measuring the actual pressure (acoustic waves) exerted on cells has not been developed. Our laboratory has discovered a potential solution to this critical problem of acoustic wave observation methods: “pressure-responsive soft materials” (molecular materials that detect pressure as molecular luminescence), which could be useful for medical diagnosis.1 We are currently conducting a large-scale search and development of pressure-responsive soft materials capable of imaging actual pressure waves exerted on cells.2-6
- Mizuno, H.; Fukuhara, G.*: Solution-State Hydrostatic Pressure Chemistry: Application to Molecular, Supramolecular, Polymer, and Biological Systems, Acc. Chem. Res. 2022, 55, 1748-1762. [Review] [Supplementary Cover]
- Kinoshita, T.; Haketa, Y.; Maeda, H.*; Fukuhara, G.*: Ground- and excited-state dynamic control of an anion receptor by hydrostatic pressure, Chem. Sci. 2021, 12, 6691-6698. [Press Release] [Highlighted in Chem-Station] [Eurekalert!]
- Ono, S.; Kinoshita, T.; Iwasaki, H.; Imai, Y.; Fukuhara, G.*: Ratiometric Chemosensors That Are Capable of Quantifying Hydrostatic Pressure Stimulus: A Case of Porphyrin Tweezers, ACS Phys. Chem Au 2024, 4, 510-521. [Front Cover]
- Kinoshita, T.; Sakamaki, D.*; Fukuhara, G.*: Multi-dimensional Dynamic Control of Supramolecular Phthalocyanine Gear: A Self-assembly System Responding to Solvent, Temperature, and Hydrostatic Pressure, ACS Omega 2024, 9, 34719-34724. [Supplementary Cover]
- Motoori, J.; Kinoshita, T.; Chai, H.; Li, M.-S.; Wang, S.-M.; Jiang, W.; Fukuhara, G.*: Dynamic Control of Chiral Recognition in Water-Soluble Naphthotubes Induced by Hydrostatic Pressure, ACS Nanoscience Au 2024, 4, 435-442. [Front Cover]
- Kinoshita, T.; Watanabe, K.; Tsurumaki, E.; Toyota, S.*; Fukuhara, G.*: Pseudohelicene chemosensor displaying ternary signaling stimulated by hydrostatic pressure and solvent, Chem. Commun. 2025, 61, 1124-1127. [Inside Back Cover]
<増幅光線力学療法グループ>
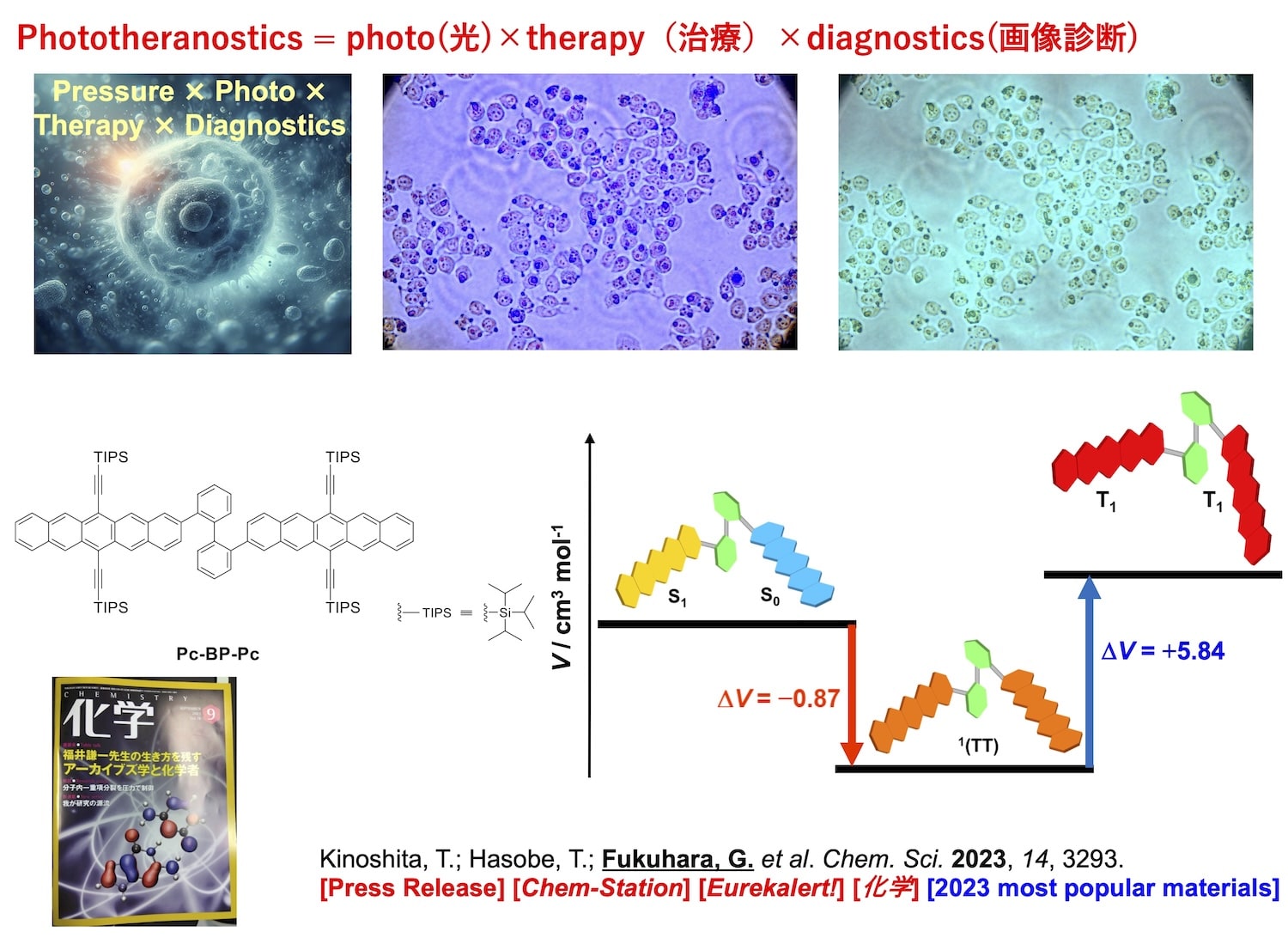
光線力学療法(PDT)はQuality of Life(QOL)の高いがん治療として注目されており、体内に光増感剤を投与後、腫瘍部分に光照射する治療法のことです。PDTは、光増感剤の投与と体外からの光照射のみで行われるため、非侵襲的な治療法として注目される一方、依然として多くの課題が残されています1。我々の研究室では、これらの課題解決を目的に、一重項分裂が進行する刺激応答性光機能ソフトマテリアルを新たな光増感剤として開発しています2-5。現在では、より高機能なPhototheranostics(光×治療×画像診断)が可能なソフトマテリアル類の開発を行っています。さらに、それらの圧力刺激によって高効率なPDTを誘起できるような光応答性マテリアルの開発へと研究を展開しています。
<Amplified Photodynamic Therapy Group>
Photodynamic therapy (PDT) has attracted attention as a cancer treatment with a high quality of life (QOL). It involves administering a photosensitizer to the body and then irradiating the tumor with light. While PDT is a non-invasive treatment, many challenges remain. 1 To address these challenges, our laboratory is developing stimuli-responsive photofunctional soft materials that undergo singlet fission as new photosensitizers. 2-5 We are currently developing soft materials capable of more advanced phototheranostics (light therapy and imaging diagnostics). Furthermore, we are expanding our research to develop photoresponsive materials that can induce highly efficient PDT using pressure stimuli.
- Matsumoto, K.; Nakagawa, K.; Asanuma, D.; Fukuhara, G.*: Recent advances in cancer detection using dynamic, stimuli-responsive supramolecular chemosensors. a focus review, Front. Chem. 2024, 12, 1478034. [Mini Review]
- Fukuchi, M.; Oyama, K.*; Mizuno, H. Miyagawa, A.; Koumoto, K.; Fukuhara, G.*: Hydrostatic Pressure-Regulated Cellular Calcium Responses, Langmuir 2021, 37, 820-826. [Supplementary Cover]
- Kinoshita, T.; Nakamura, S.; Harada, M.; Hasobe, T.*; Fukuhara, G.*: Control of intramolecular singlet fission in a pentacene dimer by hydrostatic pressure, Chem. Sci. 2023, 14, 3293-3301. [Selected in 2023 most popular materials chemistry article collection] [Press Release] [Highlighted in Chem-Station] [Eurekalert!]
- Wakako, S.; Hori, Y.; Kinoshita, T.; Saiki, T.; Qi, X.; Hasegawa, K.; Imai, Y.; Mori, T.; Nakagawa, K.; Fukuhara, G.*: Pressure-Responsive Polymer Chemosensors for Hydrostatic-Pressure-Signal Detection: Poly-L-Lysine-Pyrene Conjugates, ACS Macro Lett. 2023, 12, 1389-1395. [Supplementary Cover]
- Ogawa, R.; Kinoshita, T.; Kuwabara, T.; Sakai, H.; Harada, M.; Hasobe, T.*; Fukuhara, G.*: Critical molecular design that can actively control intramolecular singlet fission by hydrostatic pressure, Chem. Sci. 2025, 16, 20245-20254. [Press Release] [Eurekalert] [Outside Back Cover]
